◆Table of contents
ToggleIntroduction
Medical devices are products that involve human life, so extremely high quality and safety are required. High-precision and reliable molds are essential for their manufacture. In recent years, aluminum molds, which have excellent lightweightness, corrosion resistance, and thermal conductivity, have been attracting attention in the medical device field. In this article, we will introduce the importance of aluminum molds in medical devices, the required precision and safety, design and manufacturing techniques, and success stories.
Precision and safety required for aluminum molds for medical devices
Medical devices are products that affect people’s health and lives, so they require extremely high precision and safety. Aluminum molds must also meet these requirements.
Accuracy
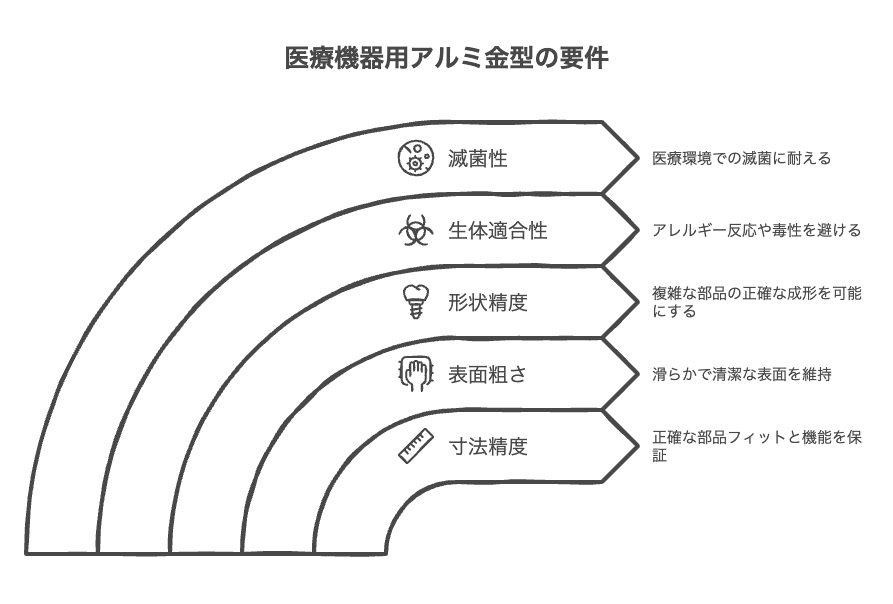
The following accuracy is required for aluminum molds for medical devices.
- Dimensional accuracy: Dimensional accuracy is required for the fitting and function of parts. For example, the piston and cylinder of a syringe and the insertion part of a catheter require high dimensional accuracy.
- Surface roughness: The surfaces of medical devices must be smooth and clean. If the surface roughness is high, bacteria may easily adhere and friction resistance may increase.
- Shape accuracy: Parts with complex shapes must be molded accurately. For example, artificial joints and implants have complex shapes and require high shape accuracy.
Safety
The following safety requirements are required for aluminum molds for medical devices.
- Biocompatibility: Considering contact with the human body, materials that do not cause allergic reactions or toxicity are selected. For example, implants and artificial joints must be made of highly biocompatible materials.
- Sterility: Materials and designs that can withstand sterilization in medical settings are required. Medical devices require sterilization because they may be contaminated by bacteria or viruses. The aluminum mold itself must also be able to withstand sterilization.
- Removal of burrs: Burrs can cause harm to medical staff and patients, so thorough removal is necessary. Burrs are small protrusions that form on the surface of medical devices and can injure the human body.
- Corrosion resistance: Corrosion caused by body fluids and disinfectants must be prevented. Medical devices must be made of highly corrosion-resistant materials because they may come into contact with body fluids and disinfectants.
To achieve both accuracy and safety
To achieve both accuracy and safety required for aluminum molds for medical devices, advanced design and manufacturing technology and a quality control system are required. Specifically, the following points are listed below.
- High-precision design: We use CAE analysis to create optimal mold structures and cooling designs.
- Precision machining technology: We use high-precision CNC machining and EDM to accurately reproduce the shape of the design drawings.
- Strict quality control: We have established a quality control system that complies with medical device standards such as ISO13485, and conduct strict inspections throughout the entire process.
Aluminum molds for medical devices are a field that requires high precision and safety, and their design and production require advanced technology and experience. However, by establishing appropriate design and manufacturing techniques and a quality control system, it is possible to provide high-quality, safe medical equipment.
Design and manufacturing technology to achieve both high precision and safety
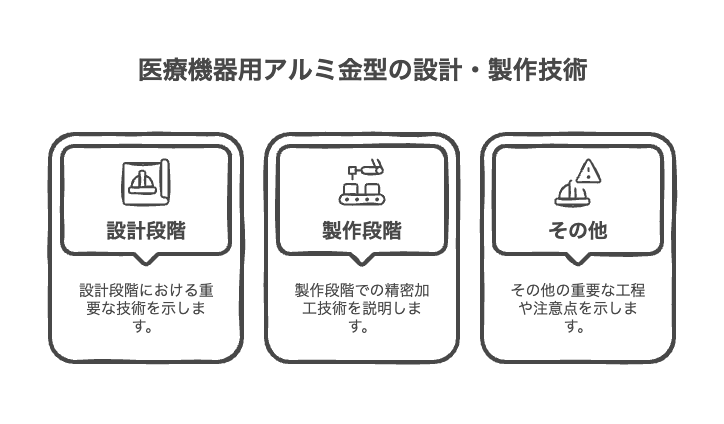
Aluminum molds for medical equipment require the conflicting requirements of high precision and safety. To meet these requirements, advanced design and manufacturing technology is essential.
Design stage
- CAE analysis: CAE (Computer Aided Engineering) analysis is a useful tool for improving the accuracy of mold design and avoiding problems. Flow analysis is used to simulate how molten aluminum alloy flows inside the mold, and problems such as poor molten alloy flow and gas entrapment are predicted. Strength analysis is used to simulate the force applied to the mold, and predicts insufficient strength and deformation of the mold. Based on the results of these analyses, optimal mold structure and cooling design are performed to realize high-precision and safe aluminum molds for medical devices.
- 3D-CAD/CAM: 3D-CAD (Computer Aided Design)/CAM (Computer Aided Manufacturing) are indispensable tools for precision mold design and machining program creation. 3D-CAD allows for accurate design of molds with complex shapes, and CAM allows for high-precision machining programs to be created based on the design data. By utilizing these tools, the entire process from design to production can be streamlined, and high-precision molds can be produced.
production stage
- High-precision CNC machining: CNC (Computer Numerical Control) machining is a technology that performs high-precision machining using computer control. By machining the mold body, cavity, and core with high precision, the dimensional accuracy of the product is improved. In addition, complex shapes and fine parts can be accurately machined.
- Precision electrical discharge machining: Electrical discharge machining (EDM) is a technology that uses electrical discharge to machine hard materials and complex shapes. It is used when machining fine and complex shapes, and contributes to improving the accuracy of molds.
- Special surface treatment: Special surface treatments such as anodizing and coating are carried out to improve the wear resistance and corrosion resistance of the mold. This can extend the life of the mold and stabilize the quality of the product.
- Quality control system: ISO13485 is an international standard for the quality control of medical devices. By establishing a strict quality control system that complies with this standard, we guarantee the quality of aluminum molds for medical devices and ensure safety.
Other
- Working in a clean room: A clean room is a special room that can minimize the contamination of airborne particles and bacteria. By manufacturing aluminum molds for medical devices in a clean room, the cleanliness of the product can be maintained and safety can be increased.
- Deburring and polishing: Burrs can lead to harm to medical workers and patients. Therefore, burrs are thoroughly removed by hand or using special tools. In addition, polishing the surface of the mold smoothes the surface roughness of the product and improves its quality.
- Cleaning and sterilization: Medical devices used in medical settings require sterilization. Therefore, aluminum molds for medical devices also need to be cleaned and sterilized so that they can withstand use in medical settings.
By combining these design and manufacturing technologies, we can create aluminum molds for medical devices that are both highly accurate and safe.
Successful case of aluminum mold for medical equipment
Case 1: Housing for medical equipment (cost efficiency, weight reduction)
- Medical equipment manufacturer: (Specific company name unknown, but assume a case using Taiyo Parts technology as an example)
- Product name: Portable medical equipment housing
- Challenge: The cost and weight of the housing were reduced. Productivity was also required to be improved.
- Solution: Adopted aluminum molds using the die-casting method. Made use of ADC12 material and made the most of the patented manufacturing process.
- Results: Achieved 80% cost reduction. Improved productivity (170 units per month). Also contributed to the weight reduction of the housing. [1, 2]
Case 2: Endoscope mouthpiece (Reflecting customer needs and market competitiveness)
- Medical device manufacturer: KSM Co., Ltd.
- Product name: Endoscopy mouthpiece
- Challenge: Improving safety and convenience during endoscopic examinations. Product development that reflects customer needs.
- Solution: Develop products that reflect customer feedback. Using aluminum molds, we have established a system that can quickly respond to small-lot production.
- Results: Achieved market success, improving safety and convenience during endoscopic examinations. [3, 4]
Case 3: Plastic hook with LED lighting (improving visual field and meeting the needs of the medical field)
- Medical device manufacturer: Yasui Co., Ltd.
- Product name: Plastic hook with LED lighting (koplight)
- Challenge: Improving visual field in the medical field. Meeting the needs of the medical field by collaborating with doctors.
- Solution: In collaboration with doctors, we developed a plastic hook with an LED light. By utilizing an aluminum mold, we aimed to provide a high-quality, low-cost product.
- Results: We created a groundbreaking product that improves vision in the medical field. [5, 6]
Case 4: High-precision well chip (High-precision molding, quality improvement)
- Medical device manufacturer: Okada Alloy Co., Ltd.
- Product name: Microfluidic chip, high-precision well chip
- Challenge: High-precision molding of microfluidic chips, etc. An accuracy of ±0.5mm was required.
- Solution: Adopting high-precision molding technology, aluminum molds using die casting and the V process.
- Results: Manufactured products with an accuracy of ±0.5 mm. Contributed to improving the quality of medical devices and gained recognition in the industry. [7, 8]
Sources:
[1] Taiyo Parts Co., Ltd. (URL: http://www.taiyoparts.co.jp)
[2] Taiyo Parts Co., Ltd. (URL: http://www.taiyoparts.co.jp)
[3] Mold Newspaper (URL: http://md-network.pj.aist.go.jp)
[7] NC Network. (URL: http://ja.nc-net.or.jp)
[8] Okada Alloy Co., Ltd. – YouTube Channel. (URL: https://www.youtube.com/watch?v=D55Xfl29kYc)
Points to note when designing and manufacturing aluminum dies for medical devices
High precision and safety are essential for aluminum dies for medical devices. When designing and manufacturing, you must pay particular attention to the following three points.
Medical device regulations
Medical devices are strictly regulated by the regulatory authorities of each country, and compliance with legal regulations is essential.
- Compliance with regulations and standards: We comply with national and regional regulations (such as the Pharmaceuticals and Medical Devices Act, FDA regulations, MDR, etc.) and the international standard ISO13485. Regulations in the export destination must also be considered.
- Permissions and traceability: Manufacturing and sales permits and manufacturing registration may be required, so cooperation with medical device manufacturers, who are our clients, is important. We will establish a system to ensure traceability and keep records.
- Documentation: We will properly create and manage documents related to design, manufacturing, and quality control in preparation for audits.
Safety
Since medical devices come into direct contact with the human body, safety is the top priority.
- Biocompatibility: Consider the biocompatibility of the molded product material, and also consider the mold material and surface treatment.
- Risk assessment: Identify risks caused by the mold and take measures to reduce the risks.
- Sterilizability: Materials and designs that can withstand sterilization are required. Ease of sterilization is considered from the design stage.
- Burring and corrosion resistance: Thorough removal of burrs is essential. Corrosion is prevented by selecting highly corrosion-resistant materials and surface treatment.
Quality control
A strict quality control system is essential to ensure stable high quality.
- Quality management system: We have established a quality management system that complies with ISO13485 and perform quality control based on documented procedures.
- Acceptance, in-process, and final inspection: Acceptance, in-process, and final inspections are carried out to prevent the occurrence of defective products.
- Measurement equipment management and records: Regular calibration and record management of measuring equipment is performed to ensure the reliability of measurements. Appropriately create and store quality control records such as design drawings, manufacturing procedures, and inspection records.
By taking the above points into consideration, medical device manufacturers and aluminum mold manufacturers can contribute to safe medical device manufacturing by working together to build technology and quality control systems.
まとめ
Aluminum molds contribute to the high precision, light weight, and low cost of medical devices, and support the manufacture of medical devices that are safe and reliable. Advanced design and manufacturing technology and a strict quality control system are essential, and as medical technology advances, the application of aluminum molds in the medical field is expected to expand.